- Projects
Menu
2020 by Universitat Leipzig
The Project
Our main aim is to evaluate 3 different point of need systems (isothermal amplification, peakPCR and rapid antigen test) for SARS-COV-2 through a multi-country blinded study and its implementation in low resource settings. Learn more about our projects.
The consortium consists of 3 European Partners (coordination and assay development) as well as 7 North and Sub-Saharan partners (diagnostic evaluation). Learn more about who we are.
The Project
International collaboration
Building cross border collaboration involving 9 countries from Europe, North and Sub-Saharan Africa.
Capacity building
Building capacity for safe handling of BSL-3 pathogens using the glove box.
Suitcase lab implementation
Implementation of the suitcase lab for rapid detection of SARS-COV-2.
Expected Impact
COVID-19 has emerged as a new viral disease in late 2019 and has rapidly spread and developed into a global pandemic with severe health and economic impact. Although real-time RT-PCR is currently used as the standard method for SARS-COV-2 molecular diagnosis, it requires a well-established laboratory, specifically trained personnel, is time-consuming (2-5 hours) and thus involves high costs altogether. Therefore, the need of other rapid and simple diagnostic approaches maintaining the high performance of RT-PCR is of utmost urgency.
peakPCR
In the past 3 years, Prof. Stark´s at the Swiss Federal Institute of Technology, Switzerland (ETH) working group has designed and constructed a completely new PCR instrument (called “peakPCR”) with a proprietary metallic sample holder, built with inexpensive and easily available components and therefore at an extremely competitive price (Figure 1). Thanks to the low reaction volume required by the sample holder, the PCR amplification can be performed faster and generate significant reagents savings.
[…]
The Team
This project is funded by the EDCTP and the BRCCH. This project is a collaboration between partners in Ghana, Senegal, Madagascar, Nigeria, DR Congo, Sudan and Uganda, where the field activities will take place, and partners in Germany, France and Switzerland involved with development and refinement of the SARS-COV-2 rapid diagnostic tests.
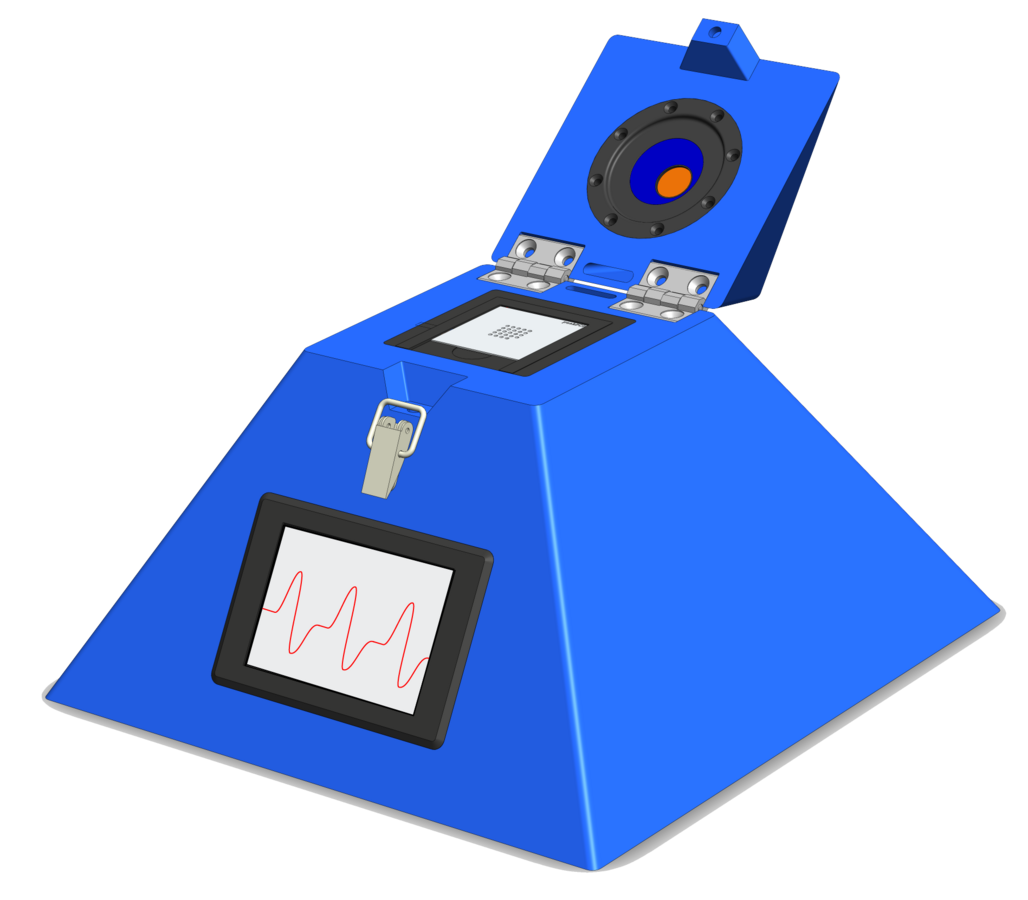
diaxxoPod (RUO)

Our in-vitro diagnostic test cartridge based on rapid Polymerase Chain Reaction (PCR) amplification technology. This cartridge can reliably detect and distinguish the mutants from UK (20I/501Y.V1, B.1.1.7 – VOC-202012/01) as well as the South African mutation (B.1.351 lineage).
The test cartridge is aimed to improve the public health situation by rapidly enabling the detection of variants of concern. The cartridge is designed for qualitative detection of variants of interest of the SARS-CoV-2 Viral RNA in saliva samples and it works in conjuction with diaxxoPCR.
The test cartridges are sold with all the necessary reagents pre-loaded in the reaction wells. The use of the test cartridges is therefore extremely simple:
diaxxoPod

The test cartridges are sold with all the necessary reagents pre-loaded in the reaction wells. The use of the test cartridges is therefore extremely simple:
We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. By clicking “Accept”, you consent to the use of ALL the cookies.